Lentiviral Vector Platform
A templated approach created by scientists with 30 years of experience.
Take the next stepEnd-to-end LVV services
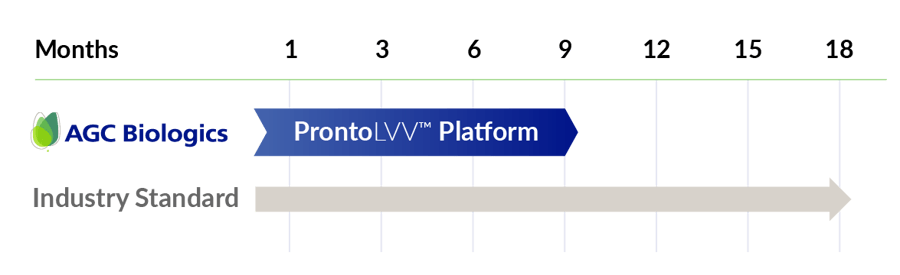
Get to GMP in half the time
ProntoLVV can help achieve your GMP goals in 9 months, helping accelerate your path from gene to the clinic!
Analysis on the promise of plug-and-play vector platforms
Explore how you can cut your timeline to cGMP in half using proven, templated processes.
ProntoLVV fact sheet
Learn more about our capabilities with our lentiviral vector platform.
View Fact SheetFill out the form below to access this research article.
Product yields & network performance
ProntoLVV offers both affordable costs on your first batch and ideal yields you need at any stage.
The platform is backed by a network of available capacity, high-quality materials and supplies.
Quality
GMP quality is at the center of everything we do. From pre-clinical through commercial, AGC Biologics has the knowledge and global regulatory expertise to help you meet your goals.
The science and processes behind ProntoLVV™ has been developed over 30 years. That makes this platform even more unique, as it is qualified by major regulatory authorities and has already been used to bring multiple commercial products to market.

Peace of mind
We ease your path to commercialization through a new one-platform-fits-all approach. Bring us your gene of interest, and let our team of 500+ highly experienced cell & gene professionals take care of the rest.
When & where you need us
We pride ourselves on being "Glocal." That means we are global and can serve you in your local market. With sites in North America and Europe, our viral vector services can meet your need anywhere in the world.