Your End-to-End ADC Solution
Powered by Proveo™

Proveo™ provides complete and efficient solutions for antibody drug conjugates (ADCs)
Proveo™ leverages AGC Biologics' specialized expertise in monoclonal antibody production, with Cerbios' proficiency in bioconjugation and linker-payload, while Oncotec provides aseptic fill and lyophilization of the ADC drug product.
Got a project? Let's talk
Fill out the form below to access the Proveo™ info sheet
Combining the expertise of 3 industry leaders:
mAb Development & Manufacturing
With 30+ years of experience in recombinant protein manufacturing, AGC Biologics provides process development, clinical supply, and commercial cGMP manufacturing and is approved for commercial production by the FDA, EMA, HC, PMDA, and ANVISA.
mAb capabilities
HiPo Linker-Payloads & Conjugation
Cerbios-Pharma SA provides 25+ years of experience in high-potency APIs and conjugation. Located in Lugano, Switzerland, Cerbios provides manufacturing of cGMP clinical batches, process validation and commercial API manufacturing inclusind full CMC support to its world-wide partners.
Aseptic Fill with Lyophilization
Oncotec Pharma Produktion GmbH is a reliable partner in the production and development of aseptically produced cytostatic drugs and brings 25+ years of experience with small and large scale filling line with lyophilization for high potent ADCs.
Your All-in-One ADC Solution
- Combined strength and expertise of three industry leaders into one integrated solution
- World-class cGMP commercial manufacturing facilities with presence in three continents
- FDA, EMA, Swissmedic and PMDA approved with an exceptional regulatory track record
- Successful development of 200+ biologics
- DS and DP contract manufacturing for more than 20 companies
- Leaders in implementation of innovative technologies and solutions that accelerate time to market
- Extensive experience in regulatory support for IND and NDA
Visit the official Proveo page
Providing full development and manufacturing of ADCs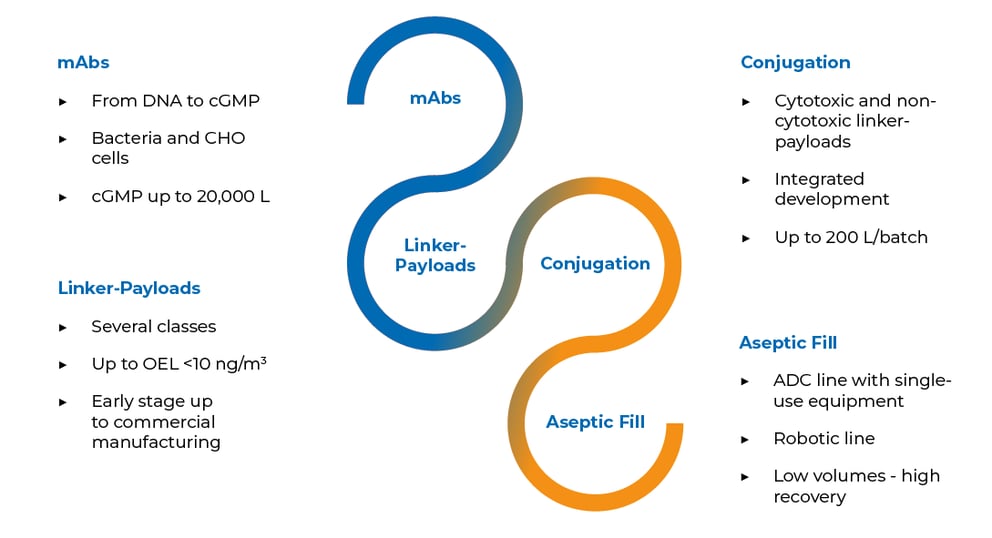
Compress your ADC timelines - DNA to fill & finish in just 15 months

Services include:
- Cell line development
- Process development for: mAb, linker-payload, conjugation
- Analytical method development and validation
- Clinical and commercial cGMP manufacturing
- Quality control and product release
- ICH stability studies
- Regulatory support






